Oropharyngeal squamous cell carcinoma (OPSCC) secondary to high-risk human papilloma virus (HPV) infection is on the rise. This malignancy caused by remote high-risk HPVinfection, usually HPV-16, has changed the way we think about head and neck cancer and our evaluation of the patient with a neck mass. From 1988 to 2004 the incidence of HPV-related OPSCC increased by more than 200 percent, and during the same interval, the incidence of non-HPV-related cancer decreased by 50 percent. Population-based estimates show upwards of 70 percent of mucosal squamous cell carcinoma of the head and neck are now secondary to high-risk HPV infection.1 These cancers are most commonly seen in younger (40s-50s) male patients without traditional risk factors of tobacco and ethanol use. From a clinical standpoint, these cancers are generally characterized by smaller, more asymptomatic primary tumors in the palatine or lingual tonsils with early and often bulky nodal disease.2 Despite the presence of bulky nodal disease, these patients have a much improved prognosis over those with traditional tobacco- and alcohol-related malignancy employing both surgical and non-surgical management.3
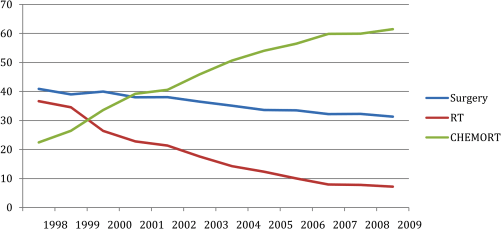
Interestingly, over this same 20-year interval where we have seen an increase in HPV-related OPSCC, we have also seen a decrease in surgical management for oropharyngeal carcinomas.3.5 Non-surgical therapy appeared to offer a less morbid alternative with equivalent outcomes. The landmark Veterans Affairs’ (VA) laryngeal cancer study published in 1991 supported cisplatin-based induction chemotherapy followed by radiation therapy to preserve the larynx.4 Later trials demonstrated improved outcomes with concurrent platinum-based chemoradiation therapy for both laryngeal and non-laryngeal sites with meta-analysis demonstrating an improved outcome with concurrent versus neoadjuvant drug therapy.5 For much of this recent history, we were not aware of the growing proportion of our patients who had HPV-related oropharyngeal carcinomas. In 2000, researchers at Johns Hopkins University published one of the first and most compelling manuscripts describing this new disease entity of HPV-induced oropharyngeal carcinoma.6 Still, another 5-10 years would pass before the implications of this disease entity became thoroughly understood. In hindsight, it has become clear that much of the perceived improvement in outcomes with concurrent platinum-based chemoradiation for oropharyngeal carcinomas were in fact secondary to the growing proportion of HPV-induced carcinomas rather than through significant improvements in efficacy of therapy.
In 2007, surgeons at the University of Pennsylvania published the first series of patients with oropharyngeal carcinomas treated with transoral robotic surgery ( TORS ).7 Additional series followed and based on efficacy and low morbidity, the FDA approved TORS for management of oropharyngeal carcinomas in 2009. Over the next five years, evidence has mounted relating to the efficacy of TORS for early T-stage oropharyngeal carcinomas, exactly the population with HPV-positive disease. Treatment based solely on imaging results in both upstaging and downstaging errors.8 Upfront surgery with TORS and selective neck dissection allows adjuvant therapy decision making based on pathologic results of TORS and neck dissection. This in turn can allow a reduction in the field and dose of radiotherapy required and often eliminate the need for chemotherapy. As a result, TORS is now being studied for HPV-positive oropharyngeal carcinoma in a randomized controlled trial.
This trial, ECOG 3311, focuses on the use of TORS to de-escalate the intensity of therapy for patients with HPV-positive OPSCC. Although concurrent chemoradiation therapy is extremely effective in this group, the implications of long-term treatment morbidities such as progressive pharyngeal fibrosis are more likely to affect this population of patients. All patients with acceptable performance status and T1-2, N1-2b OPSSC first undergo transoral resection and selective neck dissection. Those with low-risk features of negative margins and N0 or N1 neck disease are simply observed. Those with high-risk features such as positive margins — >1 mm of extracapsular spread or > 5 positive nodes — receive adjuvant concurrent chemoradiation therapy. However, the majority of patients will be in the intermediate category of clear or close margins — minimal ecs and between two and four positive nodes. These patients will be randomized to receive either 50 Gy or 60 Gy. This trial will assess two-year progression-free survival, loco-regional recurrence, and functional outcomes. Our hope is that this trial will lay a foundation for safe de-escalation of therapy for our HPV-positive patients. The University of Wisconsin Head and Neck Surgery and Radiation Oncology groups are participating in this trial and look forward to the insight into this disease that they will likely provide. Although the ultimate solution for this disease rests in population-wide acceptance and administration of the HPV vaccine to adolescent boys and girls, it is likely that this disease will play a dominant role in the care of head and neck cancer for many years to come.
Citations
1 Chaturvedi AK, Engels EA, Pfeiffer RM, Hernandez BY, Xiao W, Kim E, Jiang B, Goodman MT, Sibug-Saber M, Cozen W, Liu L, Lynch CF, Wentzensen N, Jordan RC, Altekruse S, Anderson WF, Rosenberg PS, Gillison ML. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011 Nov 10;29(32):4294-301. doi: 10.1200/JCO.2011.36.4596. Epub 2011 Oct 3.
2 Allen CT, Lewis JS, El-Mofty S, Haughey BH, Nussenbaum B. Human papilloma virus and oropharyngeal cancer: biology, detection and clinical implications. Laryngoscope. 2010;120: 1756-1772.
3 J.R. de Almeida, J.K. Byrd, R. Wu, C.L. Stucken, U. Duvvuri, D.P. Goldstein, et al. A systematic review of transoral robotic surgery and radiotherapy for early oropharynx cancer: a systematic review Laryngoscope (2007).
3.5 Temporal trends in oropharyngeal cancer treatment and survival: 1998-2009.
Chen AY, Zhu J, Fedewa S. Laryngoscope. 2014 Jan;124(1):131-8. doi: 10.1002/lary.24296. Epub 2013 Aug 5.
4 Induction Chemotherapy plus Radiation Compared with Surgery plus Radiation in Patients with Advanced Laryngeal CancerL: The Department of Veterans Affairs Laryngeal Cancer Study Group. N Engl J Med 1991; 324:1685-1690 June 13, 1991 DOI: 10.1056/NEJM199106133242402.
5 Browman GP et al. (2001) Choosing a concomitant chemotherapy and radiotherapy regimen for squamous cell head and neck cancer: A systematic review of the published literature with subgroup analysis. Head Neck 23: 579–589.
6 Gillison ML, Koch WM, Capone RB, Spafford M, Westra WH, Wu L, Zahurak ML, Daniel RW, Viglione M, Symer DE, Shah KV, Sidransky D. Evidence for a causal association between human papillomavirus and a subset of head and neck cancer. J Natl Cancer Inst. 2000 May 3;92(9):709-20.
7 Weinstein GS, O’Malley BW Jr, Snyder W, Sherman E, Quon H. Transoral robotic surgery: radical tonsillectomy. Arch Otolaryngol Head Neck Surg 2007 Dec;133(12):1220-6.
8 Walvekar RR1, Li RJ, Gooding WE, Gibson MK, Heron D, Johnson JT, Ferris RL. Role of surgery in limited (T1-2, N0-1) cancers of the oropharynx. Laryngoscope 2008 Dec;118(12):2129-34. doi: 10.1097/MLG.0b013e3181857950.