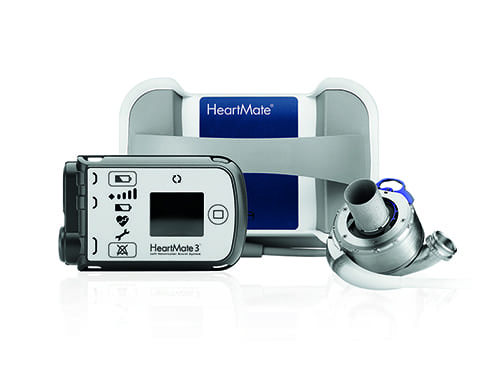
Image of the HeartMate 3 system provided courtesy of St. Jude Medical, Inc
Update: As of October 19th, the HeartMate 3 LVAD trial, which our Department is participating in, has been approved by the FDA for Destination Therapy. This is a major milestone in the trial and means this device will be available for all eligible populations. Previously, the device was restricted to our sickest patients. Now, the device will be available for other appropriate patients at UW Health facilities, as well.
Some clinical trials proceed exactly the way investigators predict. Some fall flat. But other trials exceed everyone’s expectations for success, like the Momentum 3 clinical trial of the HeartMate 3 device currently underway in our Department with the Division of Cardiothoracic Surgery. We’re proud to be the first site in Wisconsin to have tested this new device.
Helping hearts beat
When a person’s heart can no longer adequately pump blood, one treatment option is to surgically install an implantable device which manually pumps the patient’s blood. These devices include left ventricular assist devices (LVADs), which have been available to patients since the 1990s. Two types of patients can currently get LVADs: patients awaiting heart transplant, who use the device as a short-term support option, and patients not being considered for transplant who will use the device as long-term support.
HeartMate is one model of LVAD, developed by Thoratec Corporation, a St. Jude Medical Company (Abbott Laboratories). The third generation of this device, the HeartMate 3, is currently under trial at 68 sites across the nation, including UW-Madison.
Keeping the Momentum going in Wisconsin
As part of the Cardiothoracic Surgical Trials Network, the Division of Cardiothoracic Surgery participates in federally-funded cardiac research, including device trials. Because our cardiothoracic team has extensive experience with cardiac devices, Thoratec Corporation reached out to Dr. Takushi Kohmoto, Professor of Cardiothoracic Surgery, to recruit our department as the first site in Wisconsin.
“Everyone in the field knew the HeartMate 3 device was under development and was coming to clinical trial,” Dr. Kohmoto said. “We were waiting for the device and looking forward to the trial.”
The first HeartMate 3 LVAD was implanted at UW Health’s University Hospital in 2015. As of February 2018, we have enrolled 20 patients in the trial. Across the nation, over 1,000 patients have enrolled.
Multidisciplinary collaboration
Industry partners aren’t the only stakeholders needed for a successful cardiac device trial, however. UW Health’s Mechanical Circulatory Support Program is a key partner in the trial. This program is led by Dr. Ravi Dhingra, Director of UW Health’s Heart Failure and Transplant Program and Assistant Professor (CHS) in the University of Wisconsin’s Department of Medicine.
When a patient presents with heart failure, Dr. Dhingra’s team is the first to evaluate and discuss device options with the patient. Patients undergo a thorough, detailed evaluation, especially of psychosocial factors, before planning a surgery. Over the course of heart failure treatment, patients meet with social workers, device coordinators, nurses, surgeons, physical therapists, and cardiovascular medicine doctors.

“I feel so fortunate to be part of a team with Dr. Kohmoto,” Dr. Dhingra said. “He is easy to communicate with, and always available for changes in the plans of very sick patients.”
Patient results
For all involved, the best part has been the results for those in the clinical trial.
“There are several reasons this trial is so exciting,” Dr. Kohmoto said. “The device represents a really new technology compared with previous devices. It’s smaller, meaning it’s easier to surgically implant.”
“We feel very lucky to be part of this trial,” Dr. Dhingra said. “And we have short and long term results in the trial that show its success.”
To date, none of patients with the HeartMate 3 device at UW Health developed thrombosis, a serious condition where a clot forms in the pump, requiring device replacement, heart transplant, or IV anticoagulants. In the previous model of the HeartMate device, 10% of patients developed thrombosis within six months of implantation.
“For our research subjects at UW-Madison,” Dr. Dhingra said, “we were fortunate that none experienced any major complications. There were fewer readmissions related to complications or to heart failure.”
Successful results at UW-Madison included implantation in one of the research subjects with the heaviest bodyweight enrolled in the trial, a member of one of the highest risk patient populations impacted by this condition nationally.
These results were sufficiently encouraging that the FDA expanded the patient base that could access the device, allowing patients awaiting transplant to receive the HeartMate 3 LVAD starting in August, 2017. Across Wisconsin, primary care physicians sent patients to UW Health because they knew the device was available.
Preliminary results were published in The New England Journal of Medicine on February 2, 2017.
New research also found a second encouraging result: compared with patients that have older versions of these devices, those who received the HeartMate 3 in the clinical trial had fewer strokes. In research published in The New England Journal of Medicine on March 11, 2018, HeartMate 3 patients had a 10.1% occurrence of stroke compared with 19.2% in patients implanted with other versions of the device.
Clinical trials at the Department of Surgery
The Momentum 3 trial of the HeartMate 3 LVAD is in good company: every division in our Department is currently hosting a clinical trial. Last year, our Department enrolled 194 new patients in trials, and they visited us in clinic 1,250 times. We started 15 new trials and 44 trials are actively enrolling.