Check out the winners and all submissions for our Surgery Science Image Contest, held annually as part of our Research Summit.
Winner: “Normothermic Perfusion of a Donor Heart”
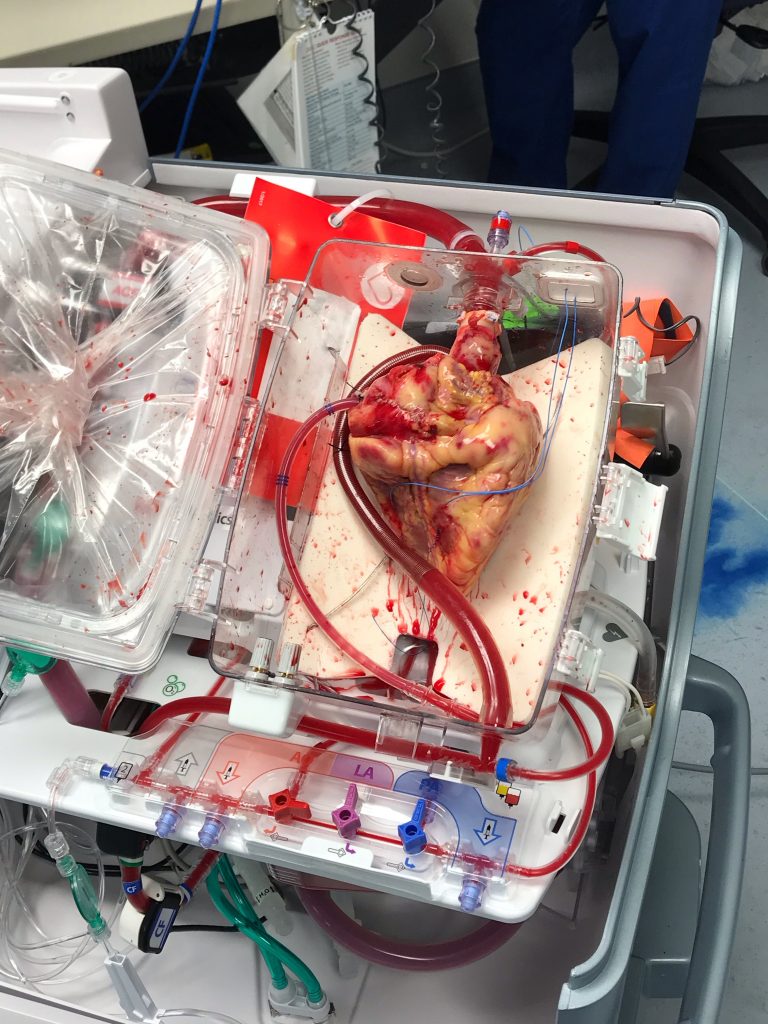
Kaelin Grant, CCRC, Research Program Manager, with the help of Jason Smith, MD, Cardiothoracic Surgery, and Stephen Devries, PA-C, Cardiothoracic Surgery
A donor heart is perfused with blood before being transplanted into a recipient. This device is part of a clinical trial in the heart transplant program in the Department of Surgery (PI Jason Smith, MD). It would allow warm blood to circulate in the heart as it travels between the donor and recipient, instead of being put in cold storage on ice.
This image was taken in the operating room at University Hospital in October 2019.
Honorable mention: Fluorescent Section of Mouse Colon
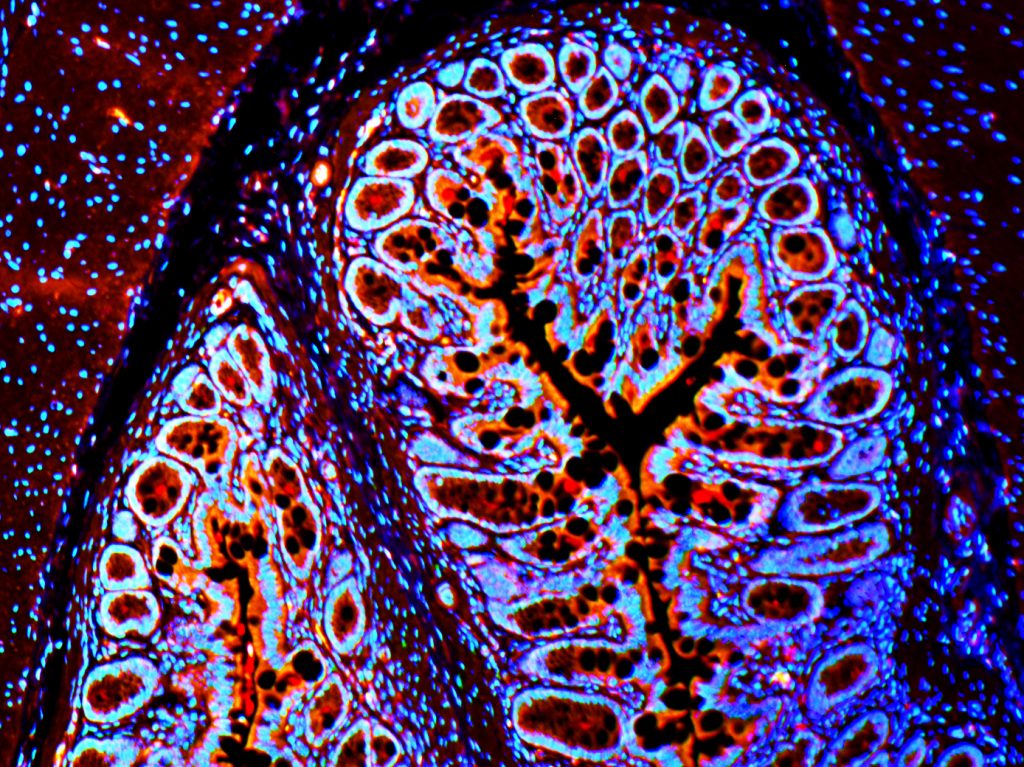
Laura Gunder, Lab Manager, Carchman Lab, Division of Colorectal Surgery
This image depicts a tissue section from a 35 week old K14E6E7 transgenic mouse. This mouse is a model of Human Papilloma Virus (HPV) in which mice develop anal cancer comparable to humans. HPV greatly increases risk of anal dysplasia and anal cancer. This photo we are reviewing the section of the colon before moving to the anus. Red represents the presence of p62, a protein that is degraded by the completion of autophagy. Our lab is reviewing autophagic induction through PI3k and mTor inhibition to prevent anal cancer in mice. This lab is led by Evie Carchman, MD.
Taken on Zeiss microscope (20x).
Honorable mention: Endovascular Treatment of a Pararenal Aneurysm
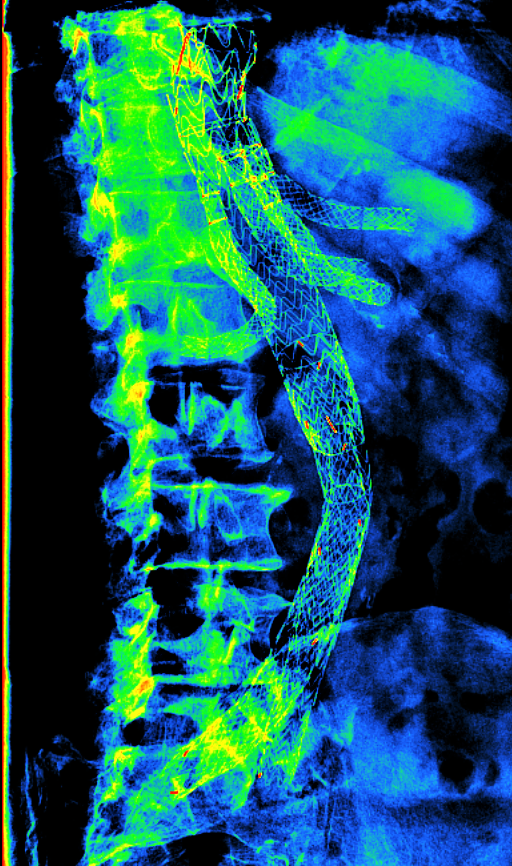
Thomas Staniszewski, Image Processor under the direction of Jon Matsumura, MD, AortaCore Aortic Imaging Lab, Division of Vascular Surgery
This X-ray image depicts a stent device in the aorta, the largest artery in the body. This device in particular has several branches that support proper blood flow to the kidneys, liver, spleen, and intestine, as well as to both legs. The X-ray has a filter that applies different colors based on the density of the structure in the body. Because of this, the device, like many bones in the body, appears to be green and blue. The device shown here is used to treat different types of aortic injuries such as an aneurysm or dissection. Since this type of stent is very complex, it takes a highly skilled surgeon to deploy the device successfully. Our lab, and many other surgeons and radiologists, analyze this type of imaging to determine if the device is functioning properly for the safety of the patient.
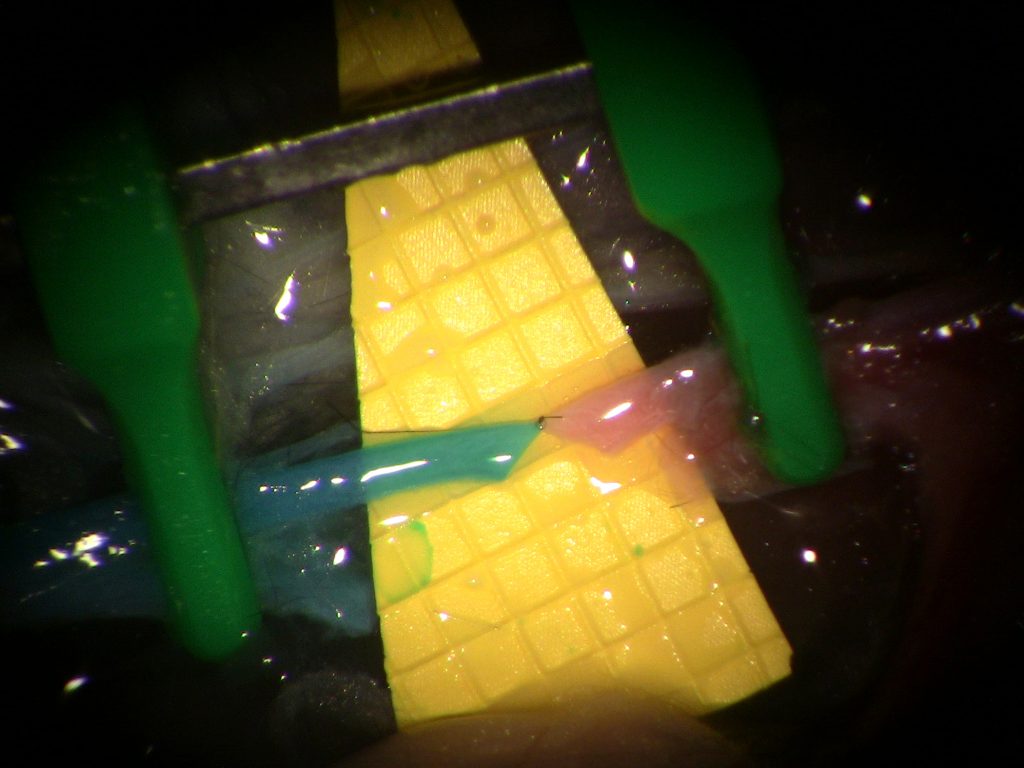
Honorable mention: “W”
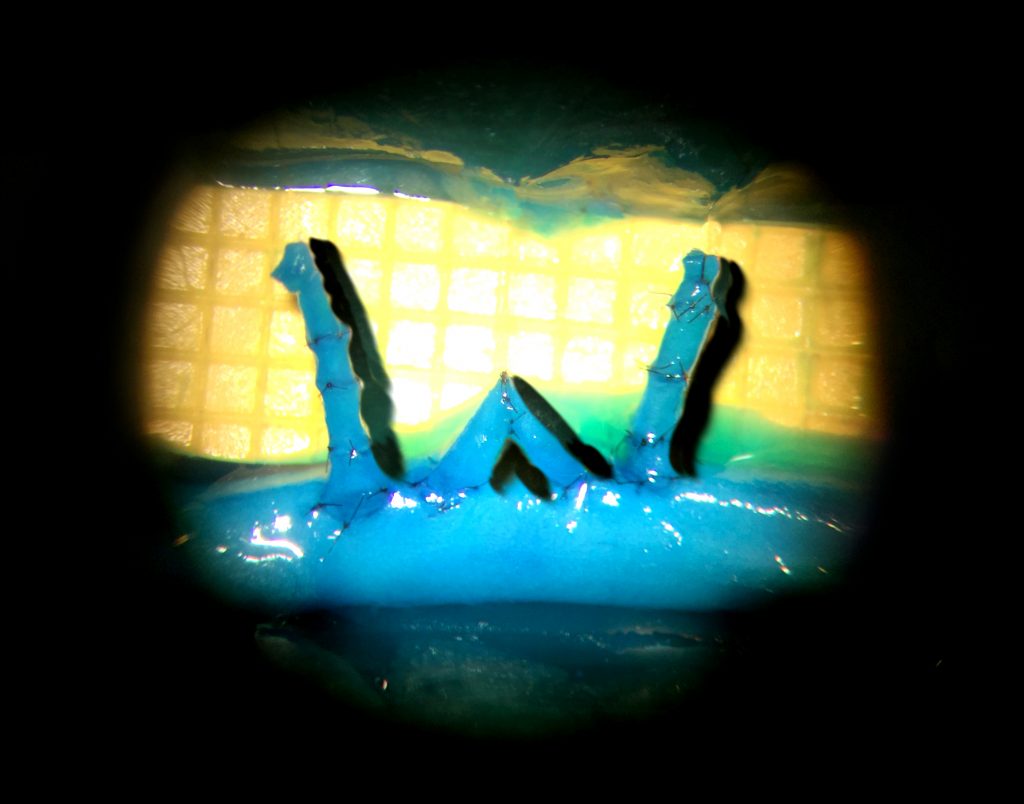
Weifeng Zeng and Ruston J. Sanchez, Microsurgical Educators and Scientists, Poore Lab, Division of Plastic Surgery
Did you know our residents can hand sew a W with their supermicrosurgical skills and a 50-micron needle? This W is made by sewing together tiny vessels (diameters less than 1mm) in our blue-blood chicken thigh model. To create this W, the residents used 60 stitches of 11-0 suture to create 7 end-to-end anastomoses and 4 end-to-side anastomoses. This image demonstrates residents in Plastic Surgery are pushing their skills to the limit during their supermicrosurgery training in Dr. Samuel Poore’s Microsurgery lab.
This image was taken with a digital camera attached to a Zeiss operating microscope during the anastomosis process. Scale: Yellow squares = 1mm2.
All submissions:
Anal Dysplasia & Cancer in Mouse Tissue
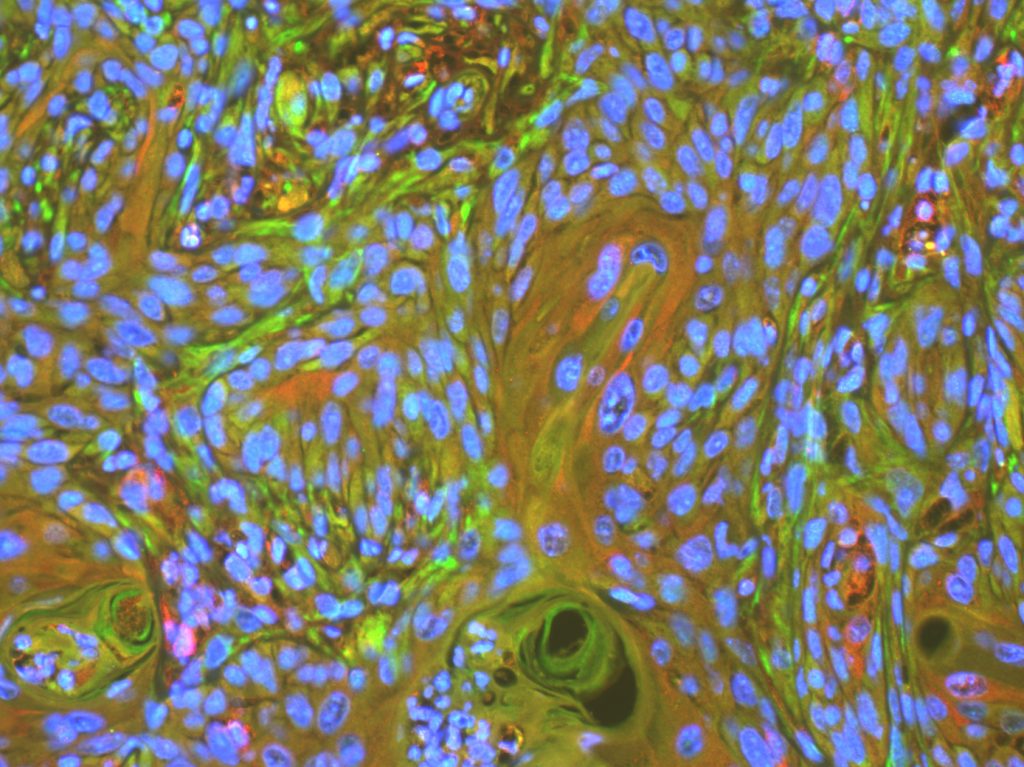
Andrew Auyeung, Research Associate, Carchman Lab, Division of Colorectal Surgery
This image depicts a tissue section from a 35 week old K14E6E7 transgenic mouse. This mouse is a model of Human Papilloma Virus (HPV) in which mice develop anal cancer comparable to humans. HPV greatly increases risk of anal dysplasia and anal cancer. This an example of squamous cell carcinoma in mouse anus tissue. Red represents the presence of p62, a protein that is degraded by the completion of autophagy and green, LC3B, which is required for the induction of autophagy. Our lab is reviewing autophagic induction through PI3k and mTor inhibition to prevent anal cancer in mice.
Taken on Zeiss microscope (20x).
The effect of HPV infection on autophagy in tissue culture
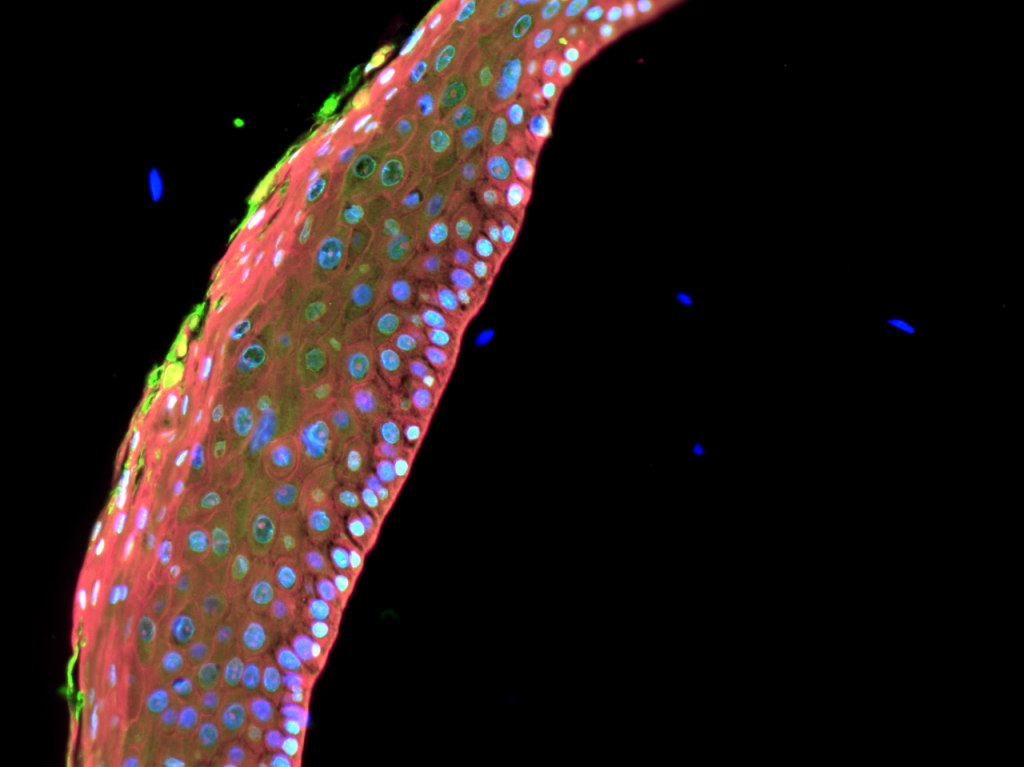
Evie Carchman, Principal Investigator, Carchman Lab, Division of Colorectal Surgery
The image of NIKS cells infected with HPV16 looking at autophagy (LC3 beta (green), p62 (red)). This image demonstrating the significant autophagic inhibition that occurs with HPV infection.
Taken on Zeiss microscope (20x).
The Noble Anastomosis
Weifeng Zeng and Aaron Dingle, Microsurgical Educators and Scientists, Poore Lab, Division of Plastic Surgery
- This image depicts the microsurgical anastomosis of the femoral vessels during a cadaveric hindlimb transplantation. The donor limb was perfused with “blue Blood”, making the donor vessels blue (left), for anastomosis to the recipient vessels (red, right).
- The joining together (anastomosis) of these two vessels in contrasting colors represents the unique collaborative environment within the UW department of surgery, bringing together international and interdisciplinary perspectives and experience to create unified healthcare solutions.
This image was taken with a digital camera attached to a Zeiss operating microscope during the anastomosis process. Scale: Yellow squares = 1mm2.
- The perfusion of “blue blood” provides distinct contrast between donor and recipient vessels during the anastomosis itself, while the patency of the anastomosis can be confirmed immediately by the perfusion of the “blue blood” into the recipient vessels.
- The “blue blood” method was originally developed in the Poore lab for training microsurgery residents using a chicken thigh model (Zeng W. et al, 2018). The addition of the “blue blood” allows for a superior class of hands on training for aspiring microsurgeons, allowing clear visualization of vessels as small as 0.5mm. In this instance, the same method was applied to rodent models of vascularized composite allografts (limb transplantation) for a recent funding applications to the Department of Defense. This image serves as part of a series of images demonstrating the ability to perform microsurgery of the highest order.
Valentine ETV
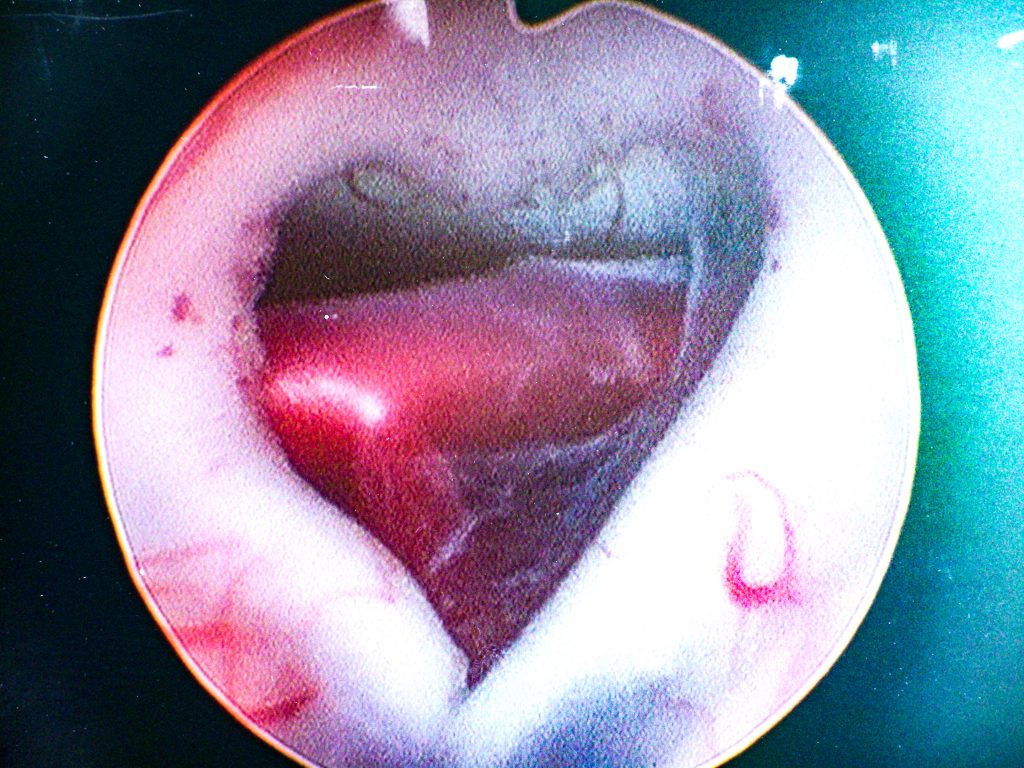
Joyce Koueik, Preliminary PGY-1 General Surgery Resident, and Theodore W. Batterman, Clinical Research Fellow, Department of Neurological Surgery, Iskandar CNS Regeneration Lab
Endoscopic third ventriculostomy (ETV) is the prevailing treatment for obstructive hydrocephalus. An endoscope is introduced into the ventricular system of the brain to create an opening in the floor of the 3rd ventricle that bypasses the obstructive lesion. This image depicts a heart-shaped third ventricular opening. The shape of the opening was unanticipated. Note the basilar artery viewed through the opening.
As a hydrocephalus researcher, this image is especially gratifying, as the ETV procedure saves patients from a lifetime of having a VP shunt system and its complications. However, exposure of the basilar artery is not trivial, as injury to the basilar artery or its perforating branches can be fatal, thus careful puncture of the membrane is critical.
Stages of the Developing Vocal Folds
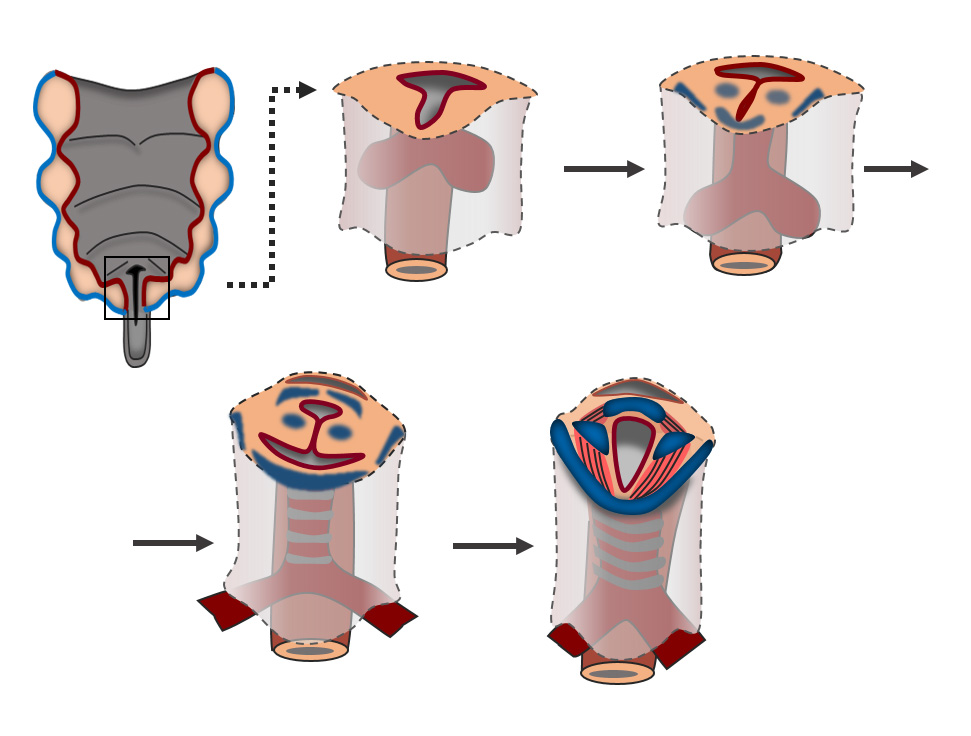
Vlasta Lungova, Associate Scientist, Thibeault Lab, Division of Otolaryngology-Head & Neck Surgery
The schematic illustration represents the stages of the murine larynx and vocal fold (VF) development. During early stages of the laryngeal embryogenesis lateral walls of the primitive laryngopharynx (LPh), the VF primordia, start to grow into the lumen and temporarily fuse to form the epithelial lamina (EL) (E11.5). This transient structure is subsequently recanalized allowing vocal folds to separate and open airways (from E13.5 – 18.5). The process of vocal fold separation is synchronized with differentiation of the vocal fold epithelium and development of laryngeal cartilages and muscles. Failure in proper vocal fold separation results in congenital laryngeal webs. Characterization of the regulatory mechanisms controlling vocal fold development is needed for understanding and evaluation of the functional prognosis in treatment approaches. Moreover, genes and signaling pathways that are critical for the larynx and VF development have a potential to be harnessed in the field of VF regenerative medicine.
The Gift of Microsurgery

Zeeda Nkana, Research Intern, Poore Lab, Division of Plastic Surgery
This is an image of a Christmas present created from 16 end-to-end anastomoses, 8 end-to-side anastomoses, and a rubber band. This creation is the result of the knowledge obtained from the 6th Annual Microsurgery Training Course, from which I learned valuable skills and has sparked my interest in microsurgery. Since this course, I have spent over 30 hours practicing my microsurgery skills and hope to one day specialize in this discipline.
Neuromuscular junctions in the posterior digastric muscle in a Ts65Dn Down syndrome mouse model
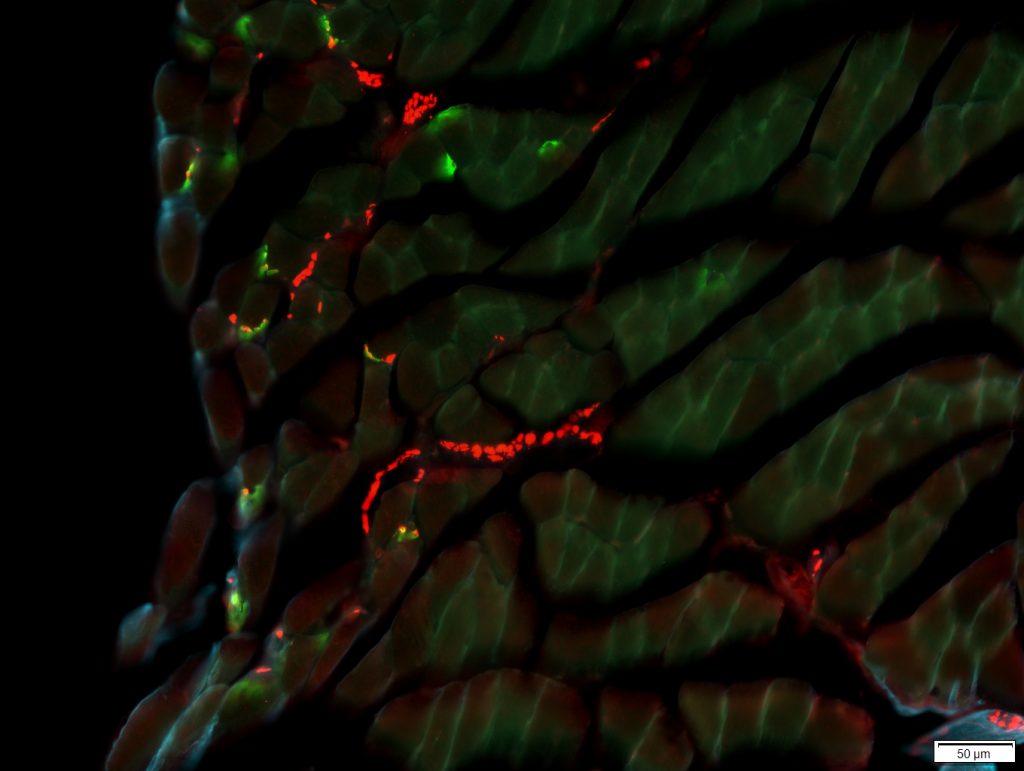
Nicole Steffes, Undergraduate Research Assistant, Connor Lab, Division of Otolaryngology-Head & Neck Surgery
The image depicts neuromuscular junctions overlap in the posterior digastric muscle, a facial muscle, of a Down syndrome mouse model. If the junctions overlap, they are innervated and appear yellow. If there is no overlap, they are denervated and appear green. Red patches with no green overlap are nerve bundles within the muscle while the blue color outlines muscle fibers. The image was captured using a digital fluorescent microscope. The muscle is a part of a preliminary study, along with other facial and limb muscles, and depicts relatively intact innervation. Imaging this muscle allows us to try to determine if swallowing and speech difficulties in Down syndrome mice might be due to neuromuscular pathological differences such as more denervation in these models.
Eye of an immunodeficient NSG mouse
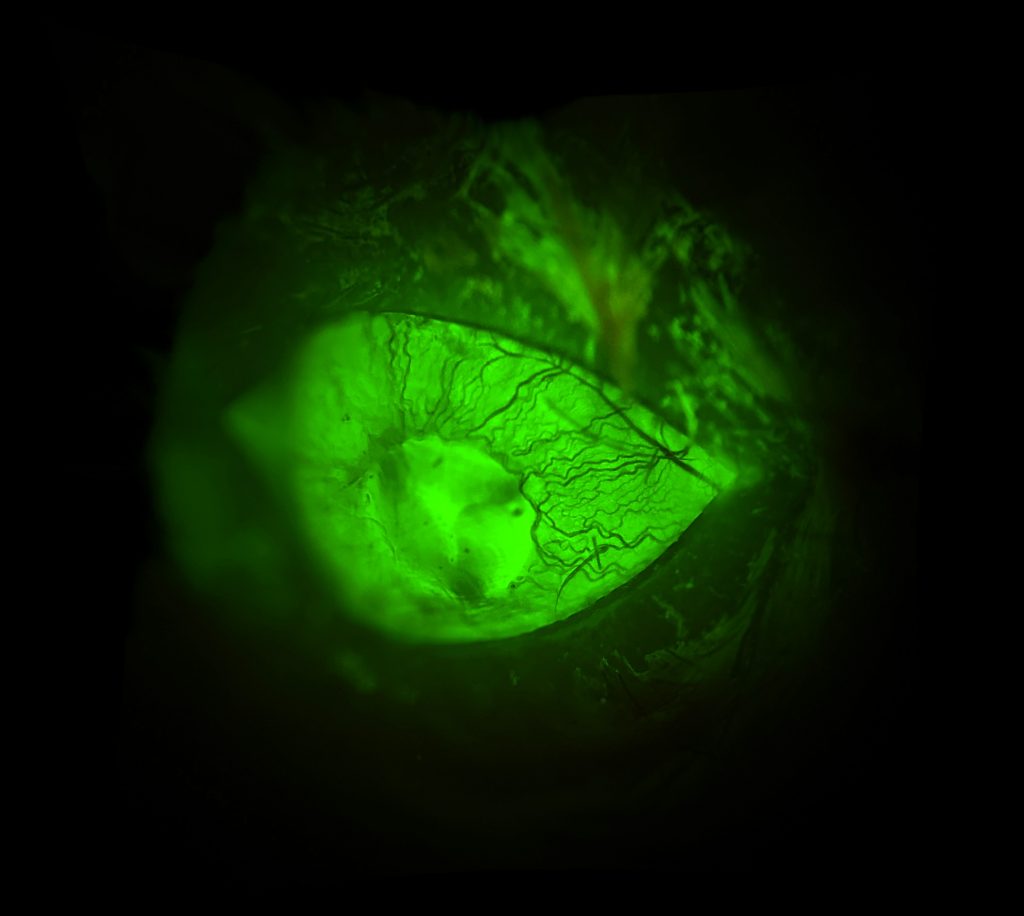
Dan Tremmel, Graduate Student, Odorico Lab, Division of Transplantation
The eye of an immunodeficient NSG mouse, in which the blood vessels of the iris are clearly visible. The mouse was injected intravenously with FITC-Dextran, a sugar polymer conjugated with a fluorescent molecule to illuminate vasculature throughout the body. Although our studies do not focus on the vasculature of the eye, we hope to use this method to assess vascularization of subcutaneous grafts as we develop novel strategies for transplanting islets and stem cell-derived beta cells.
Image taken through a 5x objective lens, with a cell phone.